Cypris Medical Suturing System
Improving Outcomes by Making Plastic Surgery Less Invasive
“I am very happy with the team effort and the contributions from industrial design, mechanical engineering and bioengineering. I am also very happy with the approach, flexibility and the huge strides we made in solving our core problem.”
Headquartered in Chicago, IL, Cypris Medical is committed to improving outcomes by making plastic surgery less invasive. The aesthetics market is growing rapidly, and to continue to meet the needs of patients, minimally invasive treatments that provide results comparable to surgery will be needed. Cypris Medical is uniquely qualified to deliver minimally invasive solutions to the market by leveraging more than 100 years combined experience in medical devices and plastic surgery.
Cypris Medical’s Xact suturing device achieved FDA clearance in April 2017 and launched officially in July 2019 following a successful evaluation period in which patients demonstrated satisfying, lasting results over two years. Procedures that use Xact are well suited to be performed without general anesthesia in out-patient settings. Procedures can be completed in less than an hour. Patients typically recover in five days or less. Comparatively, traditional facelift, browlift and necklift surgical treatments require general anesthesia and longer recovery time.

Designing Reliable, Minimally-Invasive Stitching
Traditional facelift procedures are invasive, expensive and risk complications from general anesthesia. While popular, facial injections don’t really “lift” and are only temporary. The Xact Suturing System from Cypris Medical is a minimally-invasive treatment that bridges the gap between surgery and facial injections.
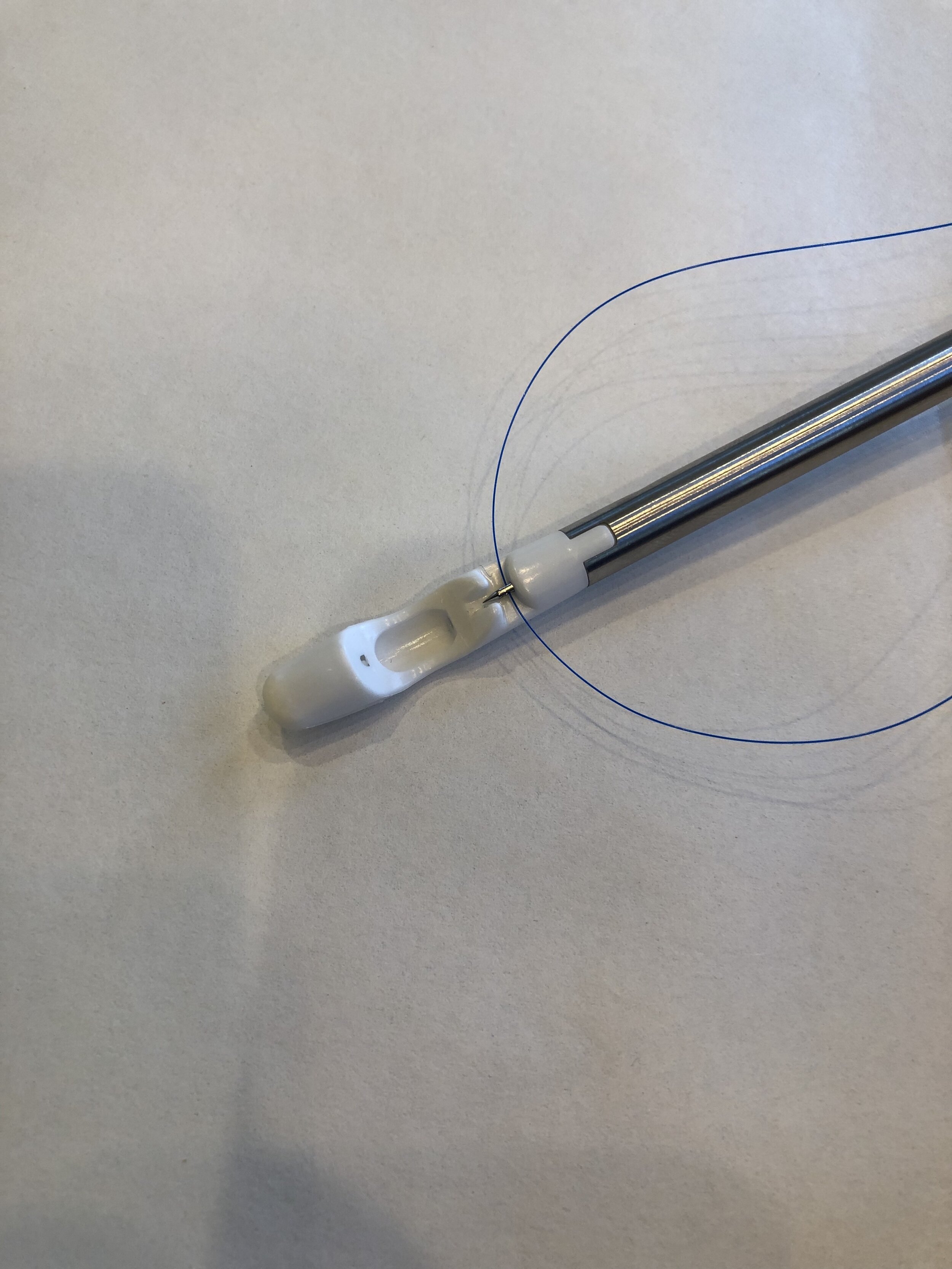
While the original Xact system was a mechanical marvel, it was plagued by occasionally capturing an inadequate amount of tissue. Market success depends on a high degree of tool reliability, and failure to secure the suture can result in an uncomfortable situation for the doctor and disappointment for the patient. Therefore, Cypris Medical CEO Dan Holton knew that throwing the blind stitch reliably was key to his game-changing product.
A Simplified Device With Improved Performance
Cypris Medical needed a second opinion on their system design. Product Creation Studio offered the needed expertise in mechanism design for interventional devices and was able to triage the potential suture pull-out causes, and quickly focus on the suture effector. Concepts were developed and rapidly tested on a bench tissue simulant.
Product Creation Studio’s expertise included:
Mechanical Engineering
Bioengineering
Industrial Design
Root Cause Analysis
Solution Ideation
Concept
Prototype
The findings were documented and a list of system improvements were transferred to the client’s development and manufacturing team. The modifications had dual benefit of device simplification and performance improvement.
Cyrpis Medical announced the commercial launch of their Xact device in July 2019. Xact is the only minimally-invasive option for effective and lasting treatment of gravitational descent in the face and neck. Read more in Cypris Medical’s launch press release.